Article Chain Arrangement and Glass Transition Temperature Variations in Polymer Nanoparticles under 3D-Confinement 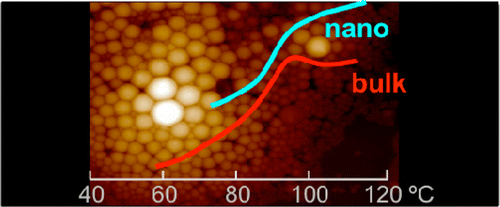 |
Polymer nanospheres with different size distributions of poly(ethyl methacrylate) are prepared by two different methods, with and without the aid of a surfactant. The calorimetric trace of these spheres shows an increase of the glass transition temperature that has been evaluated by means of an entropy model. This 3D-confinement, imposed by the nanospheres, leads to a limiting number of repeating polymer units in the sphere and thus to a reduction of the possible configuration states of the polymer chains, which is ultimately related to variations in the bulk value of the glass transition temperature. Our model is evaluated against our calorimetric measurements as well as with the data available in the literature. Good agreement between data and model is found for many cases, proving that confinement is related to reductions in entropy for these systems. Martinez-Tong et al. Macromolecules 2013, 46 (11), pp 4698–4705
|
The amorphous state can be obtained by quenching. A slow crystallization is observed at room temperature in the inner part of thick films, between glass slides or on amorphous drops (Left figures) (Tg = 24 oC). Rueda et al. Macromol. Chem. Phys. 198, 3517-28(1997) It exhibits two melting points, Tm1=120oC (A phase ) and Tm2=130oC ( C phase). A monocrystal of phase C grows as expenses of the melt of phase A (Figures on the right). Both phases differ on the molecular conformation. Rueda et al. Macromol. Chem. Phys. 198,2089-99(1997)
|
|
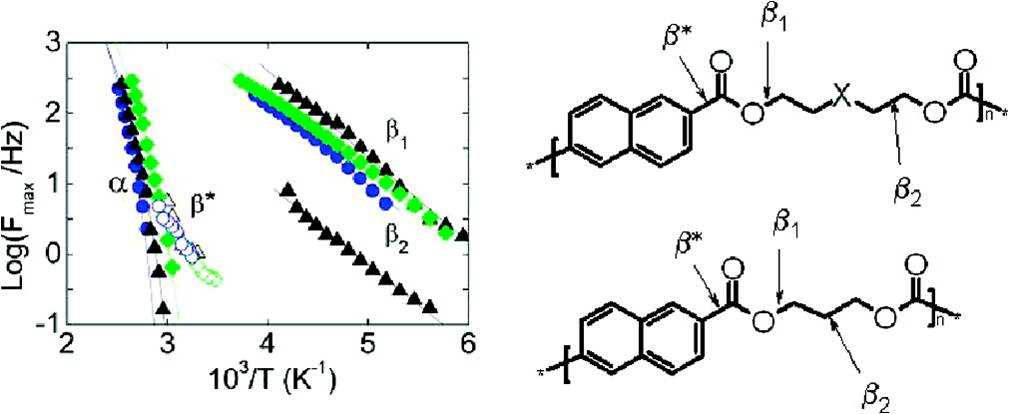
Complex subglass relaxations in aromatic polyesters with different heteroatoms |
 |